Oral Weight Loss Pill Shows 16.6% Results Rivaling Ozempic Shots
In a landmark development for obesity treatment, oral semaglutide at a 25 mg daily dose has demonstrated remarkable effectiveness in helping patients achieve significant weight loss. Published in the prestigious New England Journal of Medicine, the OASIS 4 trial results show that this once-daily pill produced an average 16.6% weight reduction—results that closely match those achieved by popular injectable medications like Wegovy and Ozempic.
Source: New England Journal of Medicine, September 18, 2025
For millions of patients struggling with obesity, this breakthrough represents something they’ve long hoped for: an effective weight loss medication that doesn’t require weekly injections. The pill offers the same powerful GLP-1 benefits in a convenient oral formulation, potentially transforming how we approach obesity treatment.
Clinical Trial Results: The Science Behind the Breakthrough
Study Design and Impressive Outcomes
The OASIS 4 trial was designed with scientific rigor that meets the highest medical standards. This randomized, double-blind, placebo-controlled study enrolled 307 adults with obesity or significant overweight across 22 clinical sites in four countries. What makes these results particularly meaningful is that the study excluded people with diabetes, focusing specifically on weight management benefits.
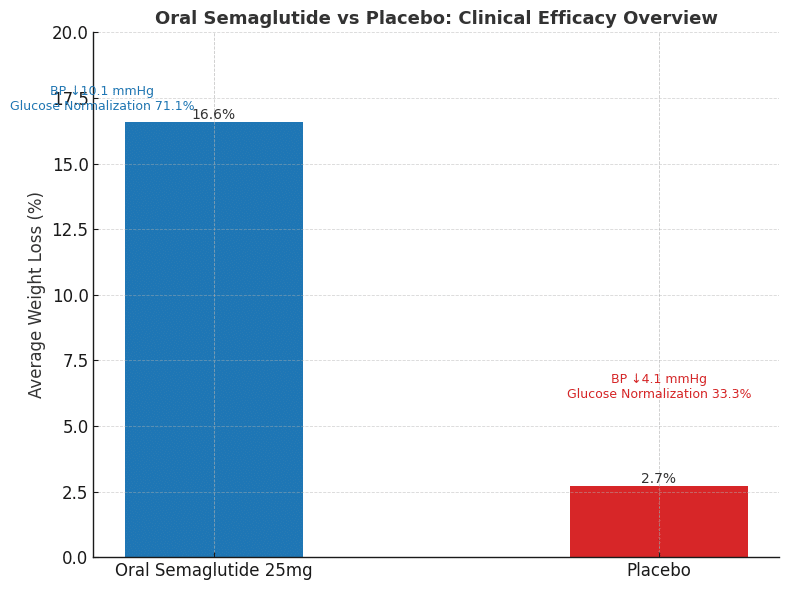
Participants received either oral semaglutide 25 mg or a placebo once daily, alongside professional lifestyle counseling, for a full 64 weeks. The results were nothing short of impressive: those taking the active medication lost an average of 16.6% of their body weight compared to just 2.7% in the placebo group.
💡 OASIS 4 KEY RESULTS
• Average Weight Loss: 16.6% with oral semaglutide vs 2.7% with placebo • Significant Results: Over one-third (34.4%) achieved ≥20% weight loss • Study Duration: 64 weeks of treatment plus 12-week dose escalation • Participants: 307 adults without diabetes • Safety Profile: Consistent with injectable GLP-1 medications • Most Common Side Effects: Nausea (46.6%) and vomiting (30.9%)
Perhaps most encouragingly, more than one-third of participants—34.4%—achieved weight loss of 20% or more, compared to just 2.9% of those receiving placebo. This level of weight reduction can be truly life-changing for people struggling with obesity-related health conditions.
Beyond Weight Loss: Comprehensive Health Benefits
The benefits extended far beyond the numbers on the scale. Participants experienced significant improvements in several key health markers that matter for long-term wellbeing. Among those with pre-diabetes at the start of the study, an impressive 71.1% achieved normal blood glucose levels, compared to just 33.3% in the placebo group.
Blood pressure improvements were equally notable. Participants who achieved 15% or greater weight loss saw their systolic blood pressure drop by an average of 10.1 mmHg, compared to 4.1 mmHg in the comparison group. Additional improvements included better cholesterol profiles, reduced inflammatory markers, and enhanced physical function in daily activities.
What This Means for Patient Access and Care
For years, one of the biggest barriers to GLP-1 therapy has been the injection requirement. Many patients simply won’t consider treatment that involves weekly self-administered shots, whether due to needle anxiety, storage concerns, or lifestyle incompatibility.
Current statistics paint a sobering picture: fewer than 2% of people with obesity in the United States receive prescription weight management medications. Fear of injections consistently ranks as a top barrier, alongside cost and access issues.
Dr. Sean Wharton, the study’s lead author and medical director of the Wharton Medical Clinic, emphasized this game-changing potential: “Oral semaglutide 25 mg builds on the proven efficacy and established safety and tolerability profile of semaglutide and represents a significant advancement in obesity treatment. People with overweight or obesity have individual preferences, and with oral semaglutide as a potential new treatment option, more of those who are not on treatment today may consider starting GLP-1 treatment.”
The convenience factor extends beyond just avoiding needles. An oral medication fits more naturally into primary care settings, potentially reducing referral delays and making treatment more accessible through telemedicine platforms and routine medical visits.
Expert Perspectives: Efficacy, Safety, and Real-World Impact
Leading obesity medicine specialists are cautiously optimistic about these results while emphasizing the importance of comprehensive care approaches.
“As recently published in The New England Journal of Medicine, the primary results from the OASIS 4 clinical trial demonstrated weight loss efficacy of investigational oral semaglutide 25 mg as a potential therapeutic option for people with obesity and overweight,” said Dr. Domenica Rubino, trial investigator and Director of the Washington Center for Weight Management and Research. “It’s exciting to see these new results from the cardiometabolic post hoc analysis, which showed that while benefits were most pronounced in people who achieved greater than 15% weight loss, clear improvements in glycemic parameters and cardiovascular risk factors were observed in patients taking oral semaglutide 25 mg, regardless of how much weight was lost.”
However, medical experts emphasize that success depends on more than just the medication itself. Long-term weight management requires ongoing lifestyle modifications, regular medical monitoring, and realistic expectations about the journey ahead.
Safety Profile and Important Considerations
The safety profile of oral semaglutide 25 mg aligned closely with what doctors already know about injectable GLP-1 medications. The most common side effects were gastrointestinal, including nausea (experienced by 46.6% of participants) and vomiting (30.9%). These effects were generally mild to moderate and tended to diminish over time as patients adjusted to the medication.
Importantly, the discontinuation rate due to side effects was relatively low at 6.9%, compared to 5.9% in the placebo group. Serious adverse events were actually less common in the treatment group (3.9%) than in the placebo group (8.8%).
Regulatory Timeline and Market Impact
Novo Nordisk submitted its New Drug Application (NDA) to the U.S. Food & Drug Administration in early 2025, with the FDA formally accepting the submission on May 2, 2025. A regulatory decision is expected by the end of 2025, potentially making this the first oral GLP-1 therapy approved for weight management.
If approved, this development could significantly reshape the obesity treatment landscape. The convenience of an oral formulation may encourage more healthcare providers to offer weight management services and make patients more willing to begin treatment.
From a market perspective, analysts predict that oral GLP-1 therapies could capture substantial market share from injectable formulations, particularly as manufacturing scales up and potentially reduces costs. However, much will depend on insurance coverage policies and pricing strategies that have yet to be announced.
Production and Availability Plans
Novo Nordisk has been preparing for potential approval by scaling up production capabilities. The company has already begun manufacturing at its North Carolina facilities, suggesting confidence in regulatory success and a commitment to avoiding the supply shortages that have plagued injectable GLP-1 medications.
This proactive approach to manufacturing could be crucial for patient access if the medication receives approval, especially given the massive unmet need in obesity treatment.
Important Safety Information and Patient Considerations

⚠️ ESSENTIAL SAFETY INFORMATION
Common Side Effects: • Nausea (46.6% of patients) • Vomiting (30.9% of patients) • Generally mild to moderate and temporary
Important Contraindications: • Personal or family history of medullary thyroid carcinoma (MTC) • Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) • History of severe pancreatitis or gallbladder disease requires caution
Dosing Requirements: • Must be taken on empty stomach with water only • Wait at least 30 minutes before eating or taking other medications • Requires gradual dose escalation over 12 weeks
Realistic Expectations: • Individual results will vary from the 16.6% average • Requires commitment to lifestyle changes • Not a short-term or “quick fix” solution
Looking Ahead: The Future of Obesity Treatment
The development of oral semaglutide represents more than just a new medication option—it signals a broader evolution in how we approach obesity as a medical condition. With effective oral treatments becoming available, obesity care may finally move from the margins of healthcare into the mainstream.
Several factors will determine the real-world impact of this advance. Insurance coverage decisions will be crucial, as will the final pricing structure that Novo Nordisk establishes. Healthcare provider education about obesity as a chronic medical condition requiring treatment will also play a vital role.
Perhaps most importantly, this development may help reduce the stigma that still surrounds obesity treatment. An oral medication may feel more “normal” to patients who have avoided seeking help due to concerns about injectable treatments or the complexity of specialized obesity care programs.
Competitive Landscape and Future Innovations
Novo Nordisk isn’t alone in developing oral GLP-1 therapies. Eli Lilly is advancing its own oral candidate, orforglipron, through clinical trials with results expected in late 2025. This competition could ultimately benefit patients through improved access, innovation, and potentially more favorable pricing.
The success of oral semaglutide may also accelerate research into other oral formulations of obesity medications, potentially expanding treatment options even further in the coming years.
Conclusion: A New Chapter in Weight Management
The OASIS 4 trial results mark a significant milestone in obesity treatment, demonstrating that effective weight loss medication can be delivered in a convenient oral form without sacrificing efficacy. With 16.6% average weight loss and meaningful improvements in overall health markers, oral semaglutide 25 mg offers genuine hope for millions of patients who have struggled with traditional weight management approaches.
As we await regulatory approval, the healthcare community is preparing for what could be a fundamental shift in how obesity is treated. For patients, this represents the possibility of accessing life-changing treatment in a more comfortable, convenient format.
The era of effective oral obesity treatment may finally be arriving—bringing with it the potential to help far more people achieve healthier weights and improved quality of life.